how to draw molecular orbital diagram of co
Use the buttons at the top of the tool to add orbitals in order of increasing energy starting at the bottom with the. How to know which molecular orbital diagram to use for heteronuclear diatomic.
What Is The Molecular Orbital Energy Diagram Of Co Quora
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules.
. Molecular Orbitals for Larger Molecules 1. BO 12 bonding e- - antibonding e- 122222 - 21 colorblue25 And this should make sense because NO is isoelectronic with CO which has a bond order of 3. Explore bonding orbitals in other small molecules.
Draw the orbital diagram for the ion Co2. Draw the orbital diagram for ion Co 2. Greater overlap greater change in.
MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works. There are 4 electrons in the outer shell of carbon and 6A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear. Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals.
The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules. The bond order is already calculated in the diagram. Find the characters of the reducible representationfor the combination of.
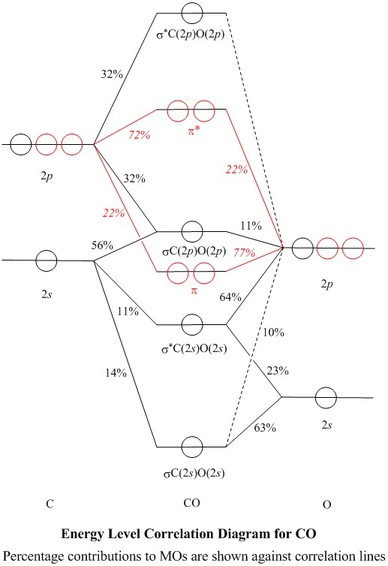
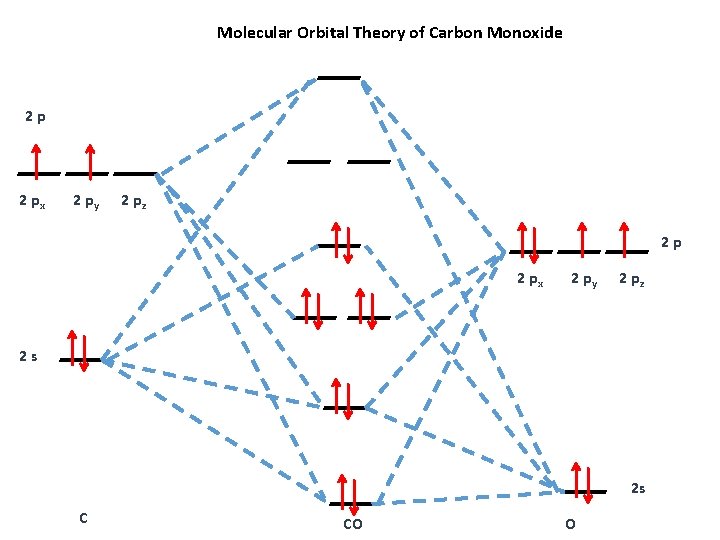
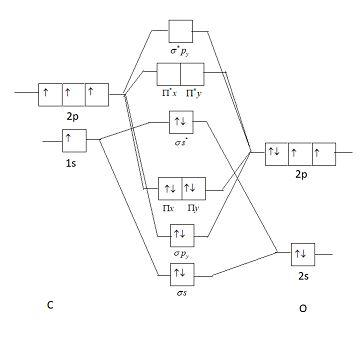
See how carbon monoxide acts as a ligand on transition metals. CO molecule has 10 valence electronsfour from carbon atom 2s²2p² and six from oxygen atom 2s²2p⁴According to molecular orbital diagram molecular orbital configuration is given as σ2s² σ2s² πx² πy² σz² πx⁰ πy⁰ σz⁰ Thus bond order 12 823. 8 - Drawing Molecular Orbital Diagrams.
σ-ML4 Tetrahedral MO Diagram e. Next article Molecular Orbital Diagram of NO. Its most important property is burning in air to give CO 2 in the combustion of fossil fuels.
Draw the orbital. CO molecule has 10 valence electronsfour from carbon atom 2s²2p² and six from oxygen atom 2s²2p⁴According to molecular orbital diagram molecular orbital configuration is given as. Hydrogen Fluorine Nitrogen Hydrogen Fluoride Carbon Monoxide Methane Ammonia Ethylene Acetylene Allene.
If I need to draw MO diagram for carbon monoxide CO how do I know which one to use as C and O both have different ones. CO is a very stable 10-valence-electron molecule isoelectronic with CN and with N 2 which has a slightly lower bond dissociation energy than CO. Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules.
Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Draw the orbital diagram for ion Co 2. All About Chemistry - July 2 2020.
The electronic configuration of carbon and oxygen atom are 1s²2s²2p² and 1s²2s²2p⁴ respectively. Depending on if it is a homonuclear case where the bonding atoms are the same or a heteronuclear case where the bonding atoms are. Watch the video solution for the question.
O_2 is well-known to be paramagnetic and it is one of the successes of molecular orbital theory. You can see that CO is not as it has zero unpaired electrons but NO is it has one unpaired electron. In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule.
Molecular Orbital Diagram of CO. The content is presented using short focussed and interactive screencast. Molecular Orbital Diagram of CO.
Point out key differences between the diagrams and use the diagram to explain why ceCO acts as a two-electron donor through carbon rather than through oxygen. D0 ions d7 ions Fe1 Ru1 Co2 Rh2 Ni3 etc. Molecular Orbital Diagrams.
The other is for AFTER nitrogen start. Assign x y z coordinates z axis is principal axis. As it is sometimes explained the statement that 4 s orbital is lower in energy than 3 d But while you fill 3 d orbital with electrons it becomes lower and lower in.
Based on the amount of orbital overlap the relative changes in energy differ going from the atomic orbital to the molecular orbital. Understandably the key difference between these molecules is that ceCO is heteronuclear and thus will have differences in energy between the molecular orbital and the atoms. If non-linear y axes of outer atoms point to central atom3.
High-spin octahedral d7 has LFSE o. It is analogous to the atomic orbital energy diagram which goes 1s 2s 2p 3s. 12-12 This video describes the molecular orbital theory diagram of CO placing emphasis on how MO theory differs for homo and heteronuclear diatomics.
Can be accommodated in the metal d orbitals. AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals. Draw the MO for O 2.
Previous article Wohl-Ziegler Bromination. σ2s² σ2s² πx² πy² σz² πx⁰ πy⁰ σz⁰. The bonding electrons are in the sigma_2s.
Well the MO diagram for O_2 is. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. The angular overlap diagrams for the molecular orbitals with high d orbital.
The purpose of MO theory is to fill in the gap for some. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. Thanking for reading this post If you find.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. Determine point group of molecule if linear use D2h and C2v instead of Dh or Cv 2. Molecular orbital energy level diagram of CO molecule can be given as.
These are UVVisible Infra-red IR and Nuclear Magnetic Resonance NMR spectroscopies. The formal bond order of CO is 3 from about one σ- bond and two π- bonds. On left and right sides of the mixed orbitals vary based on the difference in electronegativity.
Next well see that symmetry will help us treat larger. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.

Mo Diagram Of T D Cso 4 As Interaction Between A Cs Ion And An Download Scientific Diagram
Mo Diagram Of Co The Student Room
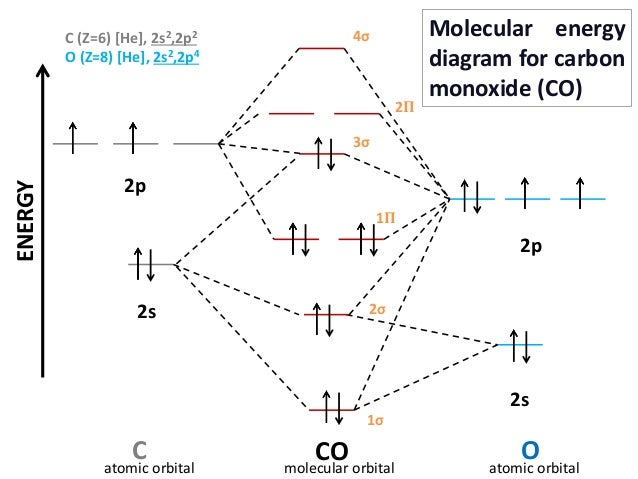
Molecular Orbital Diagram Of Co And No

Draw The Molecular Orbital Diagram For Co Based On Your Diagram Why Does Co Always Bond Through The Carbon And Not The Oxygen Atom Study Com

8 Drawing Molecular Orbital Diagrams Flux Science

Molecular Orbital Diagram Of The Co Molecule Excluding 1s Atomic Download Scientific Diagram
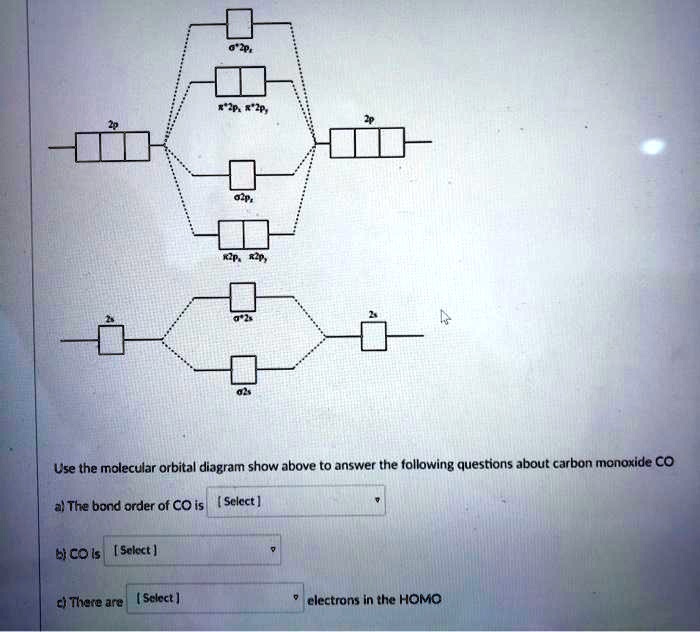
Solved Use The Molecular Orbital Diagram Show Above To Answer The Following Questions About Carbon Monoxide Co The Bond Order Of Co Is Sclect Bicois Select Ci There Are Sclect

Molecular Orbitals For Carbon Monoxide
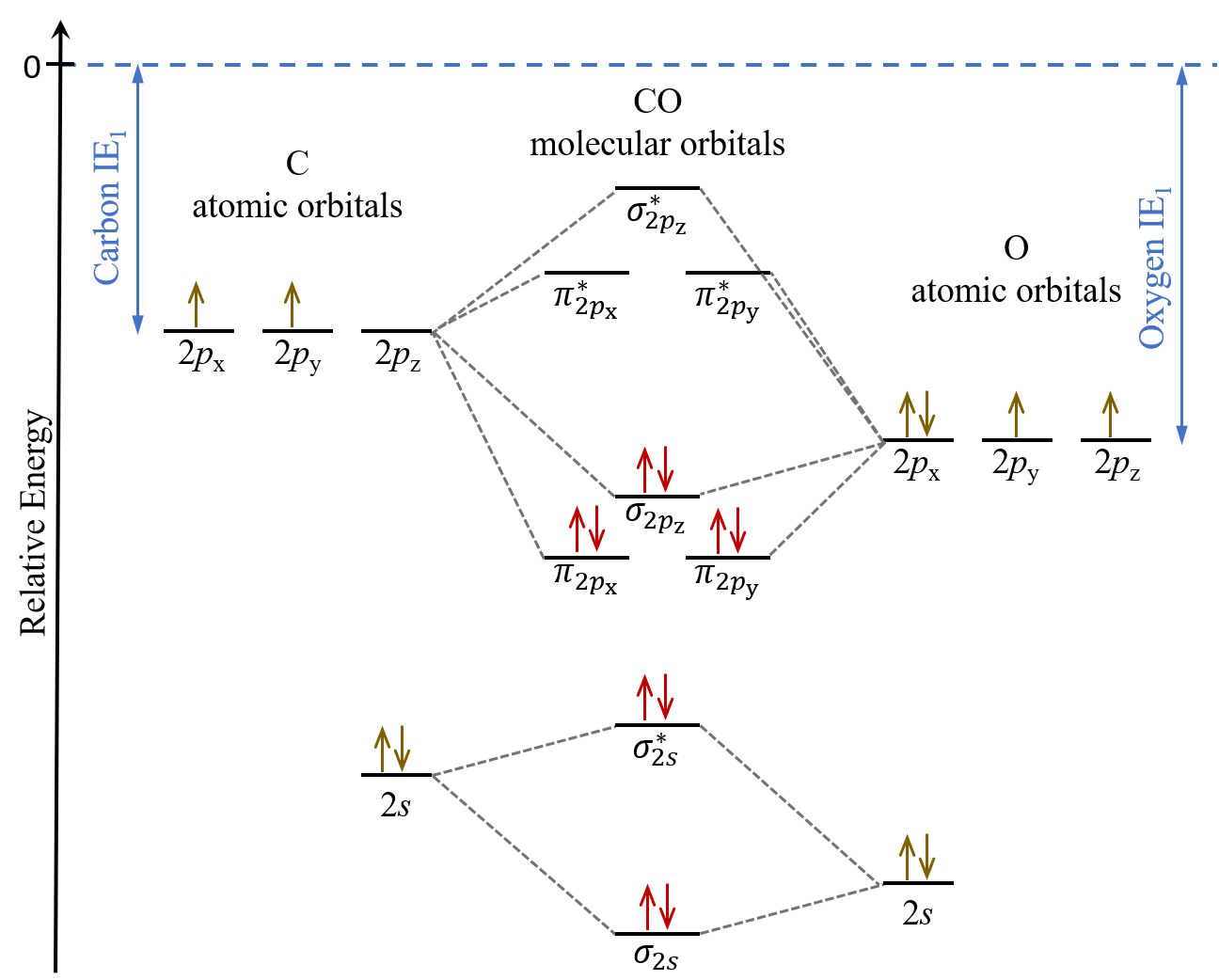
D6 5 Mos For Heteronuclear Diatomic Molecules Chemistry 109 Fall 2021

Molecular Orbitals Diagrams Of Ti H2o 6 3
Molecular Orbital Theory Or When Electrons Dont Like

Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As
The Ground State Electronic Configuration Of Co Molecule Class 12 Chemistry Cbse
Answer In Inorganic Chemistry For Lontum Rodrique 112252

Figure S6 Molecular Orbital Mo Diagram For The Valence Mos Of Ibr Download Scientific Diagram
